
Lewis Dot Structure Calculator Online Desmos
Regents Chemistry Topics | Unit 5 Bonding | | TEDED Bonding Video | Bond Polarity | Dipole Dipole Forces | | Types of Bonds | Properties of Covalent Compounds | Hydrogen Bonding | | Ionization Energy | Network Solids | van der Waals Forces | | Lewis Dot Structures-Covalent | Metallic Bonds | Like Dissolves Like | | 70 Lewis Dot Structures Videos | (AP) Valence Shell Electron Pair Repulsion VSEPR(Shapes) | | Lewis Dot Structures -Ionic | Molecular polarity | Percent Water in a Hydrate | | Properties of Ionic Compounds | Intermolecular Force |
Advanced Regents Chemistry Topics | Unit 5 Bonding | | TEDED Bonding Video | (AP) Bond Energy | Hydrogen Bonding | | Types of Bonds | Properties of Ionic Compounds | van der Waals Forces | | Ionization Energy | Properties of Covalent Compounds | Like Dissolves Like | | (AP) Electron Affinity | Network Solids | | Lewis Dot Structures-Covalent | Metallic Bonds | Percent Water in a Hydrate | | (AP) Formal Charge | (AP) Valence Shell Electron Pair Repulsion VSEPR(Shapes) (AP) Bond Angles | | 70 Lewis Dot Structures Videos | (AP) Hybridization of Orbitals (AP) Determining Hybridization in Structures | (AP)Valence Bond Theory | | Lewis Dot Structures -Ionic | Molecular polarity | | (AP)Sigma and Pi Bonds | Intermolecular Force | | Bond Polarity | Dipole Dipole Forces |
My Chemical Demonstration Videos |
|
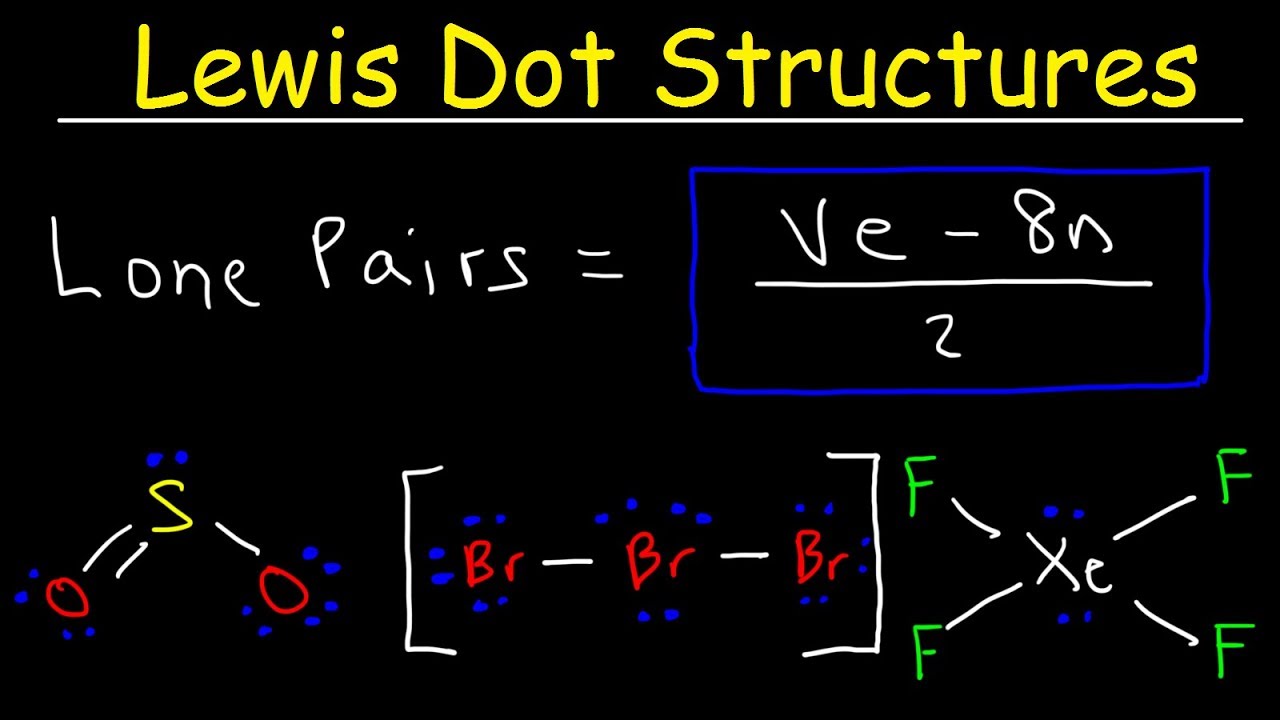
Lewis Dot Structure Calculator Online Scientific Calculator
The ChemDoodle Web Components library is a pure JavaScript chemical graphics and cheminformatics library derived from the ChemDoodle application and produced by iChemLabs. ChemDoodle Web Components allow the wielder to present publication quality 2D and 3D graphics and animations for chemical structures, reactions and spectra. Lewis Dot Structure. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron. A pair of dots between.

